Background
Diffuse large B-cell lymphoma (DLBCL) is an aggressive and highly heterogeneous lymphoma, and for high-risk group who has an International Prognostic Index (IPI) score of 3-5 it is more difficult to treat (Teras LR,2016; Ziepert M,2010). Under the standard R-CHOP-like immunochemotherapy, the 3-year overall survival (OS) rate of patients with IPI score of 0-1, 2, 3, 4-5 were 91%, 81%, 65% and 59%, respectively (Ziepert M,2010). The efficacy for high-risk population (IPI 3-5) was limited even after intensive chemotherapy, with an ORR of 74.5% and CR of 47.3% under R-CODOX-M/R-IVAC (McMillan AK,2020). Nonetheless, adding new agents to R-CHOP shows better efficacy in patients with IPI 3-5(Tilly H,2022; Nowakowski GS,2021). GCB subtype has better prognosis, but 20% of patients still relapsed after R-CHOP therapy (Nowakowski GS,2015), and no more benefit was achieved by modifying regimen with new agents like polatuzumab-R-CHP (Tilly H,2022), adding on lenalidomide (Nowakowski GS,2015) or ibrutinib (Wilson WH,2021). Selinexor is the only approved oral selective nuclear export inhibitor which demonstrated a 34% ORR in GCB subgroup as a monotherapy in relapsed/refractory DLBCL (Kalakonda N,2020). Selinexor combined with R-CHOP showed a CR rate of 58.8% and ORR of 94.1% in 17 high risk GCB DLBCL patients in a real-world setting (Zhiming Li,2023). This phase 2 study (NCT05422066) further explored the benefit and safety of Selinexor plus R-CHOP in the treatment of newly diagnosed (ND) GCB DLBCL patients with IPI 3-5.
Methods
This is a multicenter, open label, single arm phase 2 study enrolled 18~75 years old ND GCB DLBCL patients with IPI 3-5. Patients were administered selinexor 60mg QW in combination with standard R-CHOP therapy for 6 cycles (Rituximab 375 mg/m 2, cyclophosphamide 750 mg/m 2, doxorubicin 50 mg/m 2 IV, vincristine 0.5 mg/kg on day 1, prednisone 100 mg PO on days 1-5 in a 21-day cycle). The primary endpoint is complete remission (CR) rate. Secondary endpoints include objective response Rate (ORR), progression free survival (PFS), disease free survival (DFS), overall survival (OS), safety and tolerability.
Results
The median age of 15 patients was 59 years (range 25-71), with 10 males (66.7%). All patients were in advanced stage according to Ann Arbor staging system, with 13 patients in stage IV, and 2 in stage III. 11(73.3%) patients had ≥2 extranodal lesions and 9 (60%) patients had higher LDH level at baseline. 1 patient had TP53 mutation, and 6 patients (40%) had double expressor lymphoma (DEL) (Table 1).
The overall best ORR and CR were 100% and 60%, respectively. The CR rate in 6 DEL patients were 50%; Patients with ≥2 extranodal lesions reached a CR of 54.5% (n=11), while 3 out of 4 patients with≤1 extranodal lesions completely responded. The CR rates of patients with Ki-67≥80% vs<80% were 50% vs 62.5%, respectively. Median PFS and OS were not reached yet with a median follow-up of 8 months (range 2-11 months). 2 patients had progressive disease with 1 progressed after C4 and the other 1 after C6. 4 patients with viral hepatitis (3 HBV, 1 HCV) were administered both anti-tumor and antiviral therapy, and their HBV-DNA and HCV-RNA levels continuously declined (till undetectable) along with their tumor load (3 CR ,1 PR).
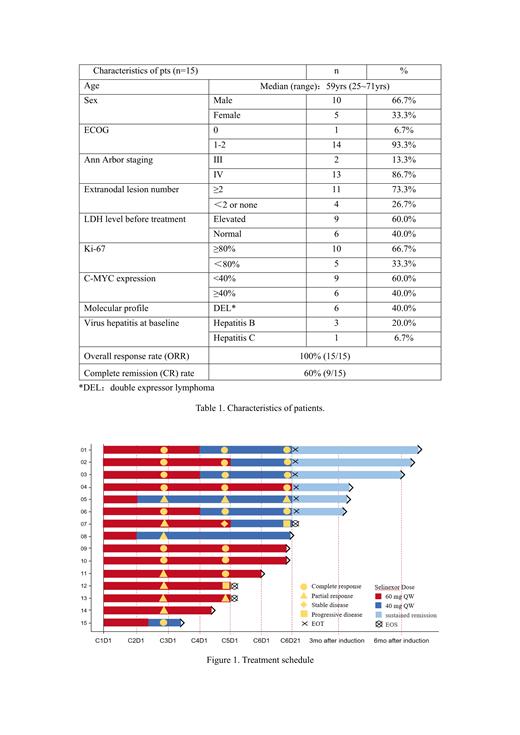
Common treatment-relate adverse events (grade 3/4, all grades) included: leukopenia (80%, 93%), neutropenia (73%, 87%), anemia (27%, 93%), thrombocytopenia (27%, 67%), lymphopenia (20%, 27%), anorexia (13%, 40%), pneumonia (13%,40%), hypertriglyceridemia (7%, 40%), diarrhea (7%, 20%). All AEs were managed with appropriate supportive care and dose adjustments. 8 out of 15 patients had a dose reduction of selinexor to 40mg (initial dose 60mg QW) due to AE, 3 in C2 and the other 5 in C4-C5. All patients were continuously treated after dose reduction, and no treatment-related deaths occurred. Overall, 6 patients completed 6 cycles of selinexor+R-CHOP treatment with sustained remission, and 6 patients were still receiving therapy at time of writing this abstract (4 in C6, 2 in C3). 3 patients ended study, 2 PD and 1 withdrew informed consent after PR at the end of C4 (Figure 1).
Conclusion
Once weekly 60mg selinexor can be safely combined with R-CHOP in ND high risk (IPI 3-5) GCB subtype DLBCL patients with ORR of 100% and CR of 60%. No viral reactivation was observed in patients with viral hepatitis with simultaneous antiviral therapy.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal